There are two classes of fluids:
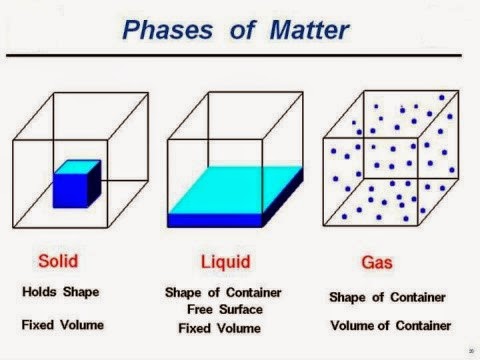
Liquids: are composed of relatively close‐packed molecules with strong cohesive forces. Liquids
have constant volume (almost in compressible) and will form a free surface in a
gravitational field if unconfined from above.
Gases: molecules are widely spaced with negligible
cohesive forces. A gas is free to expand until it encounters confining walls. A
gas has no definite volume, and it forms an atmosphere when it is not confined.
Gravitational effects are rarely concerned. Liquids and gases can coexist in
two‐phase mixtures such as steam‐water mixtures.
We can define
fluid properties and parameters, as continuous point functions, only if the
continuum approximation is made. This requires that the physical dimensions are
large compared to the fluid molecules.
- Property: means any characteristic of a system and Properties
of fluids determine how fluids can be used in engineering and technology.
They also determine the behavior of fluids in fluid mechanics.
E.g.: Pressure , Temperature
, Mass
, and Volume
.
* Properties considered to be:
Ø
Intensive Properties: Properties that are independent of the
mass of a system such as temperature, pressure, and density.
Ø Extensive Properties: Properties whose values depend
on size-or-extend-of the system such as mass, total volume, and total momentum.
Fig 2 :
Criteria to differentiate intensive and extensive properties
Ø
Specific Properties: Extensive properties per unit mass such as
specific volume
and specific total energy
.
Ø
State postulate: The state of a simple compressible system is
completely specified by two independent, intensive properties.
2.1 CONTINUUM CONCEPT OF A FLUID:
The number of molecules involved is immense, and the
separation between them is normally negligible by comparison with the distances
involved in the practical situation being studied.
Although the properties of a fluid arise from its
molecular structure, engineering problem are usually concerned with the bulk
behavior of fluids.
Under
these conditions, it is usual to consider a fluid as a continuum - a
hypothetical continuous substance – and the conditions at a point as the
average of a very large number of molecules surrounding that point within a
distance which is large compared with the mean inter molecular distance.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.